Novel MRI technique can quantify lung function
Assessing lung function is crucial for diagnosing and monitoring respiratory diseases. The most common way to do this is using spirometry, which measures the amount and speed of air that a person can inhale and exhale. Spirometry, however, is insensitive to early disease and cannot detect heterogeneity in lung function. Techniques such as chest radiography or CT provide more detailed spatial information, but are not ideal for long-term monitoring as they expose patients to ionizing radiation.
Now, a team headed up at Newcastle University in the UK has demonstrated a new lung MR imaging technique that provides quantitative and spatially localized assessment of pulmonary ventilation. The researchers also show that the MRI scans – recorded after the patient inhales a safe gas mixture – can track improvements in lung function following medication.
Although conventional MRI of the lungs is challenging, lung function can be assessed by imaging the distribution of an inhaled gas, most commonly hyperpolarized 3He or 129Xe. These gases can be expensive, however, and the magnetic preparation step requires extra equipment and manpower. Instead, project leader Pete Thelwall and colleagues are investigating 19F-MRI of inhaled perfluoropropane – an inert gas that does not require hyperpolarization to be visible in an MRI scan.
“Conventional MRI detects magnetic signals from hydrogen nuclei in water to generate images of water distribution,” Thelwall explains. “Perfluoropropane is interesting to us as we can also get an MRI signal from fluorine nuclei and visualize the distribution of inhaled perfluoropropane. We assess lung ventilation by seeing how well this MRI-visible gas moves into different parts of the lungs when it is inhaled.”
Testing the new technique
The researchers analysed 19F-MRI data from 38 healthy participants, 35 with asthma and 21 with chronic obstructive pulmonary disease (COPD), reporting their findings in Radiology. For the 19F-MRI scans, participants were asked to inhale a 79%/21% mixture of perfluoropropane and oxygen and then hold their breath. All subjects also underwent spirometry and an anatomical 1H-MRI scan, and those with respiratory disease withheld their regular bronchodilator medication before the MRI exams.
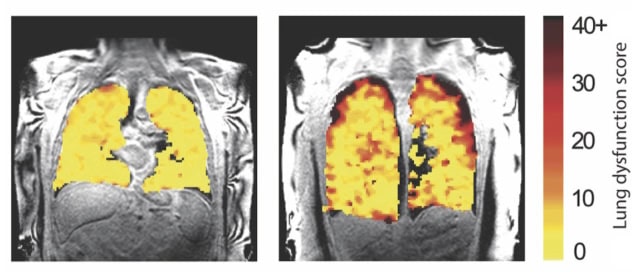
After co-registering each subject’s anatomical (1H) and ventilation (19F) images, the researchers used the perfluoropropane distribution in the images to differentiate ventilated and non-ventilated lung regions. They then calculated the ratio of non-ventilated lung to total lung volume, a measure of ventilation dysfunction known as the ventilation defect percentage (VDP).
Healthy subjects had a mean VDP of 1.8%, reflecting an even distribution of inhaled gas throughout their lungs and well-preserved lung function. In comparison, the patient groups showed elevated mean VDP values – 8.3% and 27.2% for those with asthma and COPD, respectively – reflecting substantial ventilation heterogeneity.
In participants with respiratory disease, the team also performed 19F-MRI after treatment with salbutamol, a common inhaler. They found that the MR images revealed changes in regional ventilation in response to this bronchodilator therapy.
Post-treatment images of patients with asthma showed an increase in lung regions containing perfluoropropane, reflecting the reversible nature of this disease. Participants with COPD generally showed less obvious changes following treatment, as expected for this less reversible disease. Bronchodilator therapy reduced the mean VDP by 33% in participants with asthma (from 8.3% to 5.6%) and by 14% in those with COPD (from 27.2% to 23.3%).
The calculated VDP values were negatively associated with standard spirometry metrics. However, the team note that some participants with asthma exhibited normal spirometry but an elevated mean VDP (6.7%) compared with healthy subjects. This finding suggests that the VDP acquired by 19F-MRI of inhaled perfluoropropane is more sensitive to subclinical disease than conventional spirometry.
Supporting lung transplants
In a separate study reported in JHLT Open, Thelwall and colleagues used dynamic 19F-MRI of inhaled perfluoropropane to visualize the function of transplanted lungs. Approximately half of lung transplant recipients experience organ rejection, known as chronic lung allograft dysfunction (CLAD), within five years of transplantation.
Transplant recipients are monitored frequently using pulmonary function tests and chest X-rays. But by the time CLAD is diagnosed, irreversible lung damage may already have occurred. The team propose that 19F-MRI may find subtle early changes in lung function that could help detect rejection earlier.
The researchers studied 10 lung transplant recipients, six of whom were experiencing chronic rejection. They used a wash-in and washout technique, acquiring breath-hold 19F-MR images while the patient inhaled a perfluoropropane/oxygen mixture (wash-in acquisitions), followed by scans during breathing of room air (washout acquisitions).
The MR images revealed quantifiable differences in regional ventilation in participants with and without CLAD. In those with chronic rejection, scans showed poorer air movement to the edges of the lungs, likely due to damage to the small airways, a typical feature of CLAD. By detecting such changes in lung function, before signs of damage are seen in other tests, it’s possible that this imaging method might help inform patient treatment decisions to better protect the transplanted lungs from further damage.
The studies fall squarely within the field of clinical research, requiring non-standard MRI hardware to detect fluorine nuclei. But Thelwall sees a pathway towards introducing 19F-MRI in hospitals, noting that scanner manufacturers have brought systems to market that can detect nuclei other than 1H in routine diagnostic scans. Removing the requirement for hyperpolarization, combined with the lower relative cost of perfluoropropane inhalation (approximately £50 per study participant), could also help scale this method for use in the clinic.
The team is currently working on a study looking at how MRI assessment of lung function could help reduce the side effects associated with radiotherapy for lung cancer. The idea is to design a radiotherapy plan that minimizes dose to lung regions with good function, whilst maintaining effective cancer treatment.
“We are also looking at how better lung function measurements might help the development of new treatments for lung disease, by being able to see the effects of new treatments earlier and more accurately than current lung function measurements used in clinical trials,” Thelwall tells Physics World.
The post Novel MRI technique can quantify lung function appeared first on Physics World.